BVV™ High Purity Sodium Hydroxide 99%
NSF Certified (Food Safe Chemical)
Sodium Hydroxide is a highly versatile compound that finds extensive use in various applications, including acid-base extractions. As a strong base, Sodium Hydroxide is used to adjust the pH of aqueous solutions, making it an ideal choice for separating acidic and basic compounds during extraction.
Also known as caustic soda, Sodium Hydroxide is a highly alkaline compound that is used in a wide range of applications beyond extraction including manufacturing, cleaning, and water treatment.
At BVV, we pride ourselves on offering only the highest-quality chemicals and compounds, including Sodium Hydroxide. Our Sodium Hydroxide is a premium-grade product that is manufactured to the highest standards, ensuring exceptional quality and performance for your pH adjustment needs. Our Sodium Hydroxide is NSF certified as a food safe chemical, which means it can be used in a variety of applications including drinking water and is evaluated safe for consumption.
Our product is available in a range of sizes and quantities to suit your needs, and our team is always on hand to provide expert advice and support.
High Purity Sodium Hydroxide Safety Data Sheet SDS
High Purity Sodium Hydroxide Certificate of Analysis COA

| Chemical Formula: |
NaOH |
| Molecular Weight: |
39.9971 g/mol |
| CAS Registry Number: |
1310-73-2 |
| Appearance |
White, hard (when pure), opaque crystals |
| Odor: |
Odorless |
| Density |
2.13 g/cm3
|
| Boiling Point: |
1388°C / 2530°F |
| Solubility in water: |
418 g/L (0 °C)
1000 g/L (25 °C)
3370 g/L (100 °C) |
| GHS Pictograms: |
  |
| GHS Signal Word: |
Danger |
| GHS Hazard Statements: |
H290, H302, H314
|
| GHS Precautionary Statements |
P280, P305+P351+P338, P310
|
|
UN Identification Number:
|
1823 |
|
Proper Shipping Name:
|
Sodium Hydroxide, solid |
| Transport Hazard Class: |
8 |
| Packing Group: |
II |
| DOT Placard: |
 |
What Is Sodium Hydroxide?
Sodium hydroxide, commonly known as lye or caustic soda, is a highly versatile and strong alkaline compound. It is represented by the chemical formula NaOH and consists of one sodium (Na+) ion, one hydrogen (H+) ion, and one hydroxide (OH-) ion. Sodium hydroxide is a white, odorless solid at room temperature and is highly soluble in water, producing a highly alkaline solution. It is widely used in various industries and applications, including manufacturing, chemical processes, cleaning agents, soap production, food processing, and more.
What Is Sodium Hydroxide Used For?
Sodium hydroxide (NaOH), commonly known as caustic soda or lye, has a wide range of industrial, commercial, and household uses due to its strong alkaline properties. Some of the common uses of sodium hydroxide include:
-
Chemical Manufacturing: Sodium hydroxide is a key component in the production of various chemicals, including detergents, soaps, textiles, paper, and synthetic materials.
-
Soap and Detergent Production: It is used in the saponification process to make soap and is also a crucial ingredient in many household and industrial cleaning products.
-
Food Processing: Sodium hydroxide is used to process and refine certain foods, such as in the preparation of olives, cocoa, and chocolate. It is also used for peeling fruits and vegetables.
-
Water Treatment: In water treatment, sodium hydroxide is used to adjust pH levels, remove acidity, and precipitate metals from wastewater.
-
Petroleum Industry: It is used in refining petroleum products, including the removal of impurities from crude oil.
-
Aluminum Production: Sodium hydroxide is employed in the extraction of alumina from bauxite ore and as an electrolyte in the aluminum extraction process.
-
Pulp and Paper Industry: It is used in the pulping and bleaching processes of paper manufacturing.
-
Textile Industry: Sodium hydroxide is utilized to process and dye textiles and fabrics.
-
Pharmaceuticals: It is used in the manufacture of certain drugs and pharmaceutical products.
-
Biodiesel Production: Sodium hydroxide is used as a catalyst in the transesterification process to produce biodiesel from vegetable oils or animal fats.
-
Hydrogen Production: Sodium hydroxide is used to produce hydrogen gas through the electrolysis of water.
-
Cleaning and Degreasing: It is used as a strong cleaning agent for surfaces, equipment, and industrial machinery due to its ability to dissolve oils, fats, and grease.
-
Drain Cleaning: Sodium hydroxide-based drain cleaners are used to unclog and clean drains.
-
pH Adjustment: In laboratories and industrial processes, sodium hydroxide is used to adjust the pH of solutions.
It's important to note that sodium hydroxide is a highly caustic and reactive substance, and its use should be handled with care and appropriate safety measures.
Is Sodium Hydroxide Lye?
Yes, sodium hydroxide is commonly known as lye. Lye is a strong alkaline substance that is often used in various industrial, commercial, and household applications, as mentioned earlier. It is important to handle sodium hydroxide with caution, as it can cause severe burns and irritation if it comes into contact with skin or eyes. Proper safety measures should be taken when working with sodium hydroxide or lye.
Are Sodium Hydroxide and Caustic Soda The Same Thing?
Yes, sodium hydroxide and caustic soda are the same thing. Caustic soda is a common name for sodium hydroxide, which is a strong alkaline chemical compound. Both terms refer to the same chemical substance with the chemical formula NaOH. It is used in various industrial, commercial, and household applications, including cleaning, manufacturing, and chemical processes.
What Is Sodium Hydroxide Structure?
Sodium hydroxide (NaOH) has a simple molecular structure consisting of one sodium (Na) atom, one oxygen (O) atom, and one hydrogen (H) atom. The structure of sodium hydroxide can be visualized as follows:
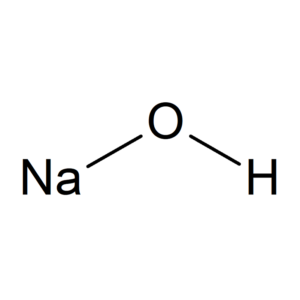
In this structure, the sodium atom (Na) is bonded to the oxygen atom (O) through an ionic bond, and the oxygen atom is bonded to the hydrogen atom (H) through a covalent bond. The hydroxide ion (OH-) is formed by the combination of the oxygen and hydrogen atoms, and it is the key component that gives sodium hydroxide its strong alkaline properties.
What Is The pH of Sodium Hydroxide?
The pH of sodium hydroxide (NaOH) is very high and strongly alkaline. Sodium hydroxide is a strong base and is commonly used to raise the pH of solutions. In its pure form, sodium hydroxide has a pH of approximately 14, which is the highest value on the pH scale. When dissolved in water, it dissociates into hydroxide ions (OH-) and sodium ions (Na+), resulting in a highly alkaline solution with a pH greater than 14. The exact pH of a sodium hydroxide solution will depend on its concentration and the amount dissolved in the solventWhat Are The Hazards of Sodium Hydroxide?
Is Sodium Hydroxide A Weak or Strong Base?
Sodium hydroxide (NaOH) is considered a strong base. It is highly soluble in water and dissociates completely into sodium ions (Na+) and hydroxide ions (OH-) when dissolved. This complete dissociation results in a high concentration of hydroxide ions in the solution, making sodium hydroxide a strong and effective source of hydroxide ions for chemical reactions. Strong bases like sodium hydroxide have a high tendency to accept protons (H+) from other substances, leading to the characteristic properties of strong bases, such as high alkalinity and the ability to neutralize acids.What Is Sodium Hydroxide?
What Are The Hazards of Sodium Hydroxide?
Sodium hydroxide (NaOH) is a caustic and highly reactive compound, and its use should be handled with care due to the following hazards:
-
Corrosive to Skin and Tissues: Sodium hydroxide is highly corrosive and can cause severe burns upon contact with the skin, eyes, and mucous membranes. It can damage and destroy living tissue upon contact, leading to chemical burns and tissue damage.
-
Inhalation Hazard: Inhaling sodium hydroxide dust, fumes, or mists can irritate the respiratory tract and cause coughing, shortness of breath, and lung irritation. Prolonged exposure to airborne sodium hydroxide particles can lead to more serious respiratory effects.
-
Eye Irritation: Contact with sodium hydroxide can cause irritation, redness, and damage to the eyes. Severe exposure can lead to permanent eye damage or blindness if not promptly treated.
-
Reactivity: Sodium hydroxide is highly reactive and can react violently with certain substances, especially acids. Mixing sodium hydroxide with acids can result in rapid heat generation, spattering, and potentially explosive reactions.
-
Environmental Impact: Sodium hydroxide is harmful to aquatic life and can lead to water pollution if released into the environment. It is important to properly dispose of sodium hydroxide-containing solutions and prevent their release into water systems.
-
Corrosion of Materials: Sodium hydroxide can corrode metals and other materials, causing structural damage and weakening of containers, pipes, and equipment.
-
Toxicity: Ingesting sodium hydroxide can cause severe internal burns, damage to the digestive system, and even death. Ingestion can lead to life-threatening medical emergencies and requires immediate medical attention.
To mitigate these hazards, it is essential to handle sodium hydroxide with proper protective equipment, such as gloves, goggles, and lab coats, in a well-ventilated area. Proper storage, labeling, and disposal procedures should also be followed to prevent accidents and environmental contamination.
How Do I Use Sodium Hydroxide Safely?
Sodium hydroxide, also known as caustic soda or lye, is a highly caustic and potentially dangerous chemical. It's essential to handle and use it safely to prevent accidents or injuries. Here are guidelines for safely using sodium hydroxide:
-
Protective Equipment:
- Wear appropriate personal protective equipment (PPE), including chemical-resistant gloves, safety goggles or a full-face shield, a lab coat or chemical-resistant apron, and closed-toe shoes with chemical-resistant soles.
-
Work in a Controlled Environment:
- Work in a well-ventilated area, such as a fume hood, to minimize exposure to fumes. Adequate ventilation helps disperse any fumes produced during handling.
-
Avoid Skin Contact:
- In case of skin contact, immediately flush the affected area with plenty of water for at least 15 minutes. Remove contaminated clothing and seek medical attention if irritation or burns occur.
-
Prevent Eye Contact:
- Wear chemical-resistant safety goggles or a full-face shield to protect your eyes from splashes. If sodium hydroxide comes into contact with your eyes, rinse them immediately with water for at least 15 minutes and seek medical attention.
-
Use Appropriate Containers:
- Use containers made of materials that are compatible with sodium hydroxide, such as glass or certain types of plastic. Avoid using aluminum or reactive metals.
-
Dilution Procedure:
- When diluting sodium hydroxide in water, always add the chemical to the water, not the other way around. Stir gently while adding to prevent splashing.
-
Label Containers:
- Properly label containers holding sodium hydroxide solutions with appropriate hazard warnings and information.
-
Handling Crystals or Pellets:
- Handle sodium hydroxide crystals or pellets with dry hands or appropriate gloves to avoid skin contact. Always use tools (e.g., scoops or spatulas) to handle solid sodium hydroxide.
-
Do Not Inhale Fumes:
- Avoid inhaling sodium hydroxide fumes. Work in a well-ventilated area, and if working with large quantities or generating fumes, use a fume hood or wear a chemical-resistant mask with appropriate filters.
-
Emergency Equipment:
- Have access to emergency equipment, such as eye wash stations and safety showers, in case of accidental exposure. Know the location of fire extinguishers and fire alarm systems.
-
Spill Response:
- In case of a sodium hydroxide spill, follow your organization's spill response procedures. Typically, this involves containing the spill, neutralizing with an acid if appropriate, and safely cleaning it up.
-
Storage:
- Store sodium hydroxide in a cool, dry, well-ventilated area, away from incompatible substances (e.g., acids, organic materials). Keep containers tightly closed when not in use.
-
Dispose of Waste Properly:
- Dispose of sodium hydroxide waste in accordance with local, state, and federal regulations. It is considered hazardous waste and should be handled and disposed of as such.
-
Training and Knowledge:
- Ensure that personnel working with sodium hydroxide are adequately trained in its safe handling, storage, and disposal. Training should include hazard recognition, first aid procedures, and emergency response protocols.
-
First Aid:
- Familiarize yourself with the appropriate first aid measures for sodium hydroxide exposure, including how to treat skin contact, eye exposure, and ingestion.
-
Medical Monitoring:
- If workers are regularly exposed to sodium hydroxide, consider implementing a medical monitoring program to track their health and detect early signs of exposure-related issues.
Always follow the safety guidelines and procedures established by your organization or regulatory authorities when working with sodium hydroxide. Remember that sodium hydroxide is a highly caustic substance, and improper handling can result in severe burns or other injuries. Prioritize safety and take appropriate precautions to protect yourself and others from potential hazards associated with sodium hydroxide.